Abstract
Introduction. Biopsy of affected tissue is required for lymphoma diagnosis at onset and relapse and to plan an adequate treatment. Open incisional biopsy is traditionally the method of choice, with an accuracy of approximately 100%. Nevertheless, it requires hospitalization, availability of an operating room and sometimes general anesthesia and is associated with several drawbacks (morbidity, surgical complications, tumor contamination of surrounding tissues). The development of ultrasound and computed tomography (CT)-guided fine-needle biopsies has almost overcome these disadvantages. However, a variable proportion of non-diagnostic procedures is reported, leading to an accuracy that ranges between 50% and 80%. Functional imaging, such as fluorodeoxyglucose (FDG) positron emission tomography (PET)/CT is a procedure which can potentially be used to drive biopsy to the most metabolically active area within a lymph node or to extranodal masses which sometimes show no morphologically detectable changes on CT scan.
Methods. This trial started at the beginning of 2016 and is still ongoing. One hundred patients with suspect lymphoma at onset or relapse are expected to be enrolled in 3 years, provided they show FDG-avid findings. Patients are excluded if pregnant, breastfeeding or in case a fine-needle PET/CT-guided biopsy is contraindicated. Patients are also excluded if a superficial and easily accessible lymph node is appreciated. Primary objective of the trial is to assess the diagnostic accuracy of the combined PET and CT-guided approach in driving a fine-needle biopsy. Diagnostic accuracy will be compared to published data concerning conventional imaging. Specimen adequacy (length of the specimen, amount of neoplastic infiltration, amount of fibrous or bony tissue) will also be evaluated. The trial is supported by the Italian Association for Cancer Research (Associazione Italiana per la Ricerca sul Cancro, Progetto AIRC IG 2015 Id 17781).
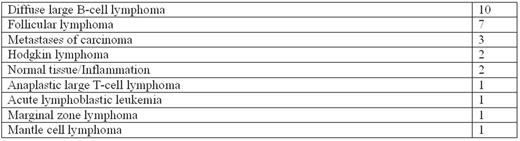
Results. Data are available for the first 32 patients enrolled (15 patients with a suspect of relapsed lymphoma and 17 with the suspect of newly onset of disease). Thirty-four procedures have been performed, 29 of them on an outpatient basis. Three procedures (8.8%) were interrupted because of unbearable pain, but could be successfully repeated in 2 cases without any complications. Pain was however always transient and never required pharmacological interventions. Biopsy target was a lymph node in 19 cases and an extranodal site in 13 (bone tissue in 8 cases, soft tissue in 3, liver and kidney in 1 each). Median standard uptake value (SUVmax) of target lesions was 11.5 (range 4.9-37.7). Insufficient samples were obtained in 9.7% of cases (3 patients), whereas in all other instances the tissue was considered adequate to formulate a diagnosis (table), including fluorescence in situ hybridization and molecular analyses, where appropriate. Mean sample length was 10 (± 1) mm (range 3-30 mm, standard deviation ± 6 mm). The mean amount of affected tissue in collected samples was 56 (standard deviation ± 33%) and the mean proportion of fibrosis or bone was 37% (standard deviation ± 32%). No severe adverse events were reported during or after each procedure and no patients required intensive monitoring after the intervention.
Conclusions. Patients can benefit from a minimally invasive procedure which allows a timely and accurate diagnosis of lymphoma at onset or relapse. Cost and time savings will be evaluated once enrolment is fully completed.
Zinzani: Celgene, Roche, Janssen, Gilead, Takeda, BMS, MSD, Servier, Sandoz, Mundipharma: Honoraria; Merck: Consultancy, Other: Advisory board; Celgene, Janssen, Gilead, Roche, Takeda, BMS, MSD, Sandoz, Servier, Mundipharma: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal